
C2h5oh Lewis Dot Structure
In order to draw the lewis structure of C2H5OH, first of all you have to find the total number of valence electrons present in the C2H5OH molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom). So, let's calculate this first. Calculation of valence electrons in C2H5OH For Carbon:
C2h5oh Lewis Dot Structure
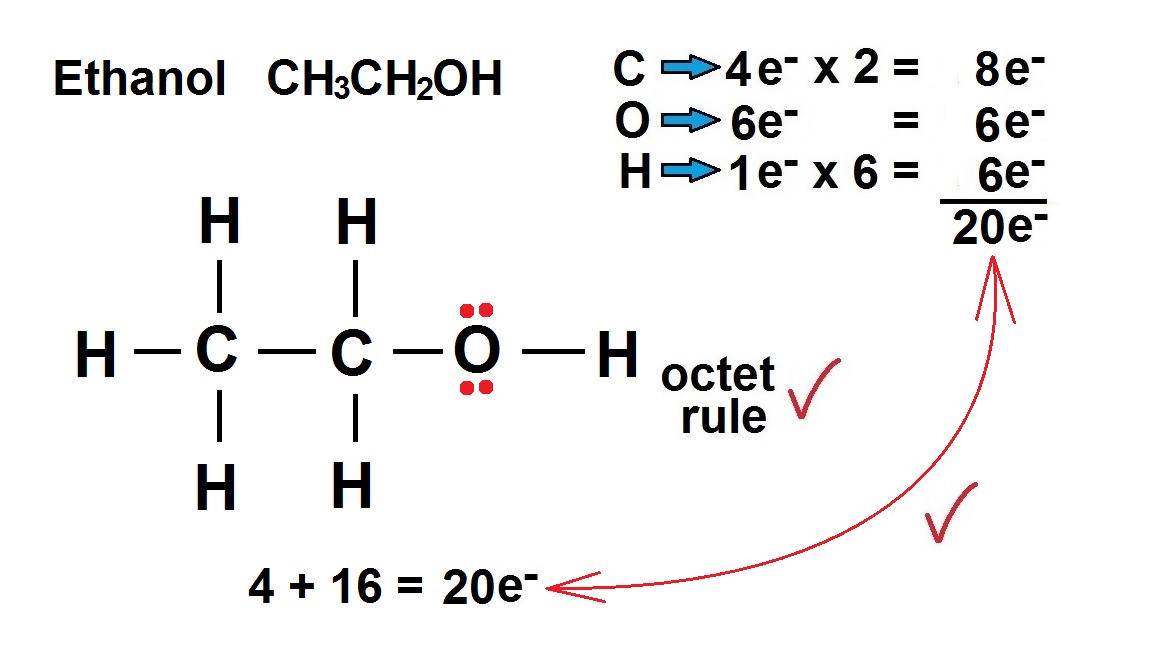
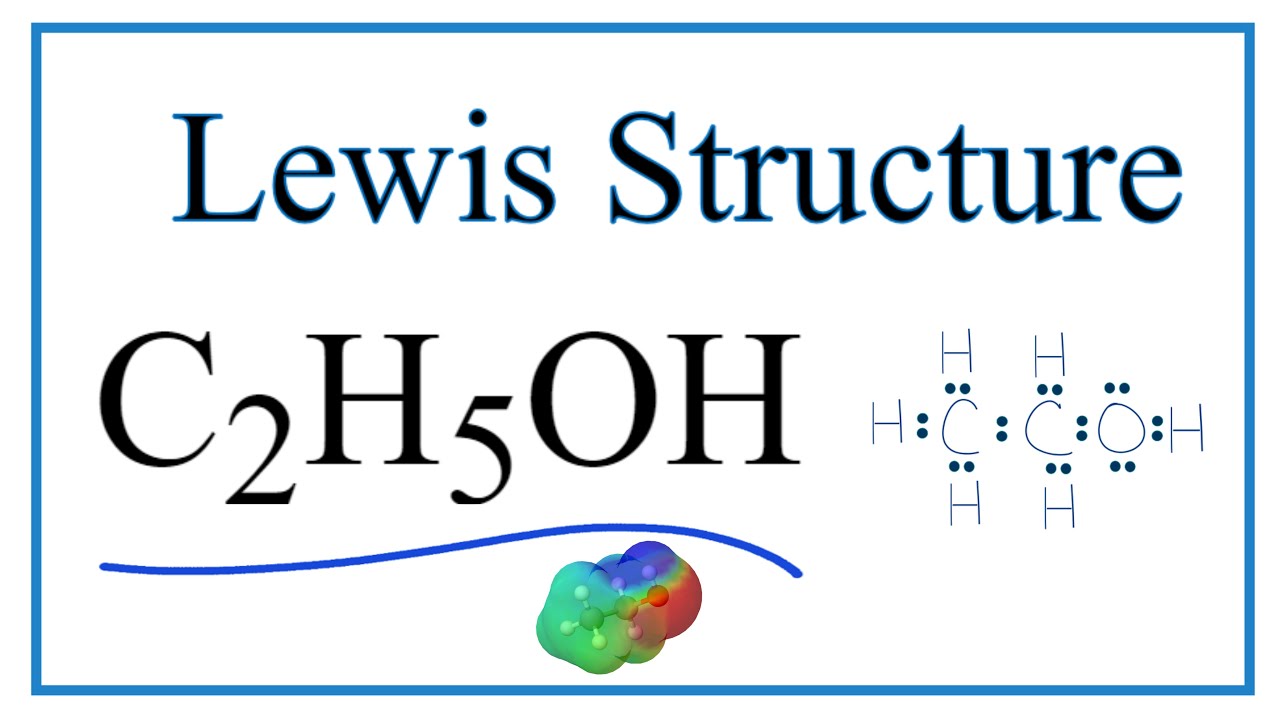
CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom. In the ethanol Lewis structure, there are five C — H bonds, one C — C bond, one C — O bond, and one O — H bond. And on the oxygen atom, there are two lone pairs. CH3CH2OH Lewis Structure: How to Draw the Lewis Structure for CH3CH2OH

C2h5oh Lewis Dot Structure
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.

C2h5oh Lewis Dot Structure
Lesson 4: Dot structures and molecular geometry. Drawing dot structures. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Worked example: Lewis diagram of xenon difluoride (XeF₂) Exceptions to the octet rule. Counting valence electrons.

C2h5oh Lewis Dot Structure
Steps for drawing the Lewis dot structure of C2H5OH 1. Count the total valence electrons in C2H5OH The very first step while drawing the Lewis structure of C 2 H 5 OH is to calculate the total valence electrons present in its concerned elemental atoms.

C2h5oh Lewis Dot Structure
Draw a Lewis structure for ethanol (C 2 H 5 OH), then answer the following questions about the structure: Number of valence electrons: Number of bonding electrons: Number of non-bonding electrons: Tries 4/99 Previous Tries Identify the fault, if any, in each of the following Lewis structures for ethanol.If more than one answer appl A Incorrect: Wrong molecular formula.

C2h5oh Lewis Dot Structure
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
C2h5oh Ethanol Molecule Royalty Free Vector Image Free Nude Porn Photos
Lesson summary FAQs Activities What is general formula of ethanol? Ethanol has the following chemical formulas: C2H5OH, C2H6O, CH3-CH2-OH. Each of these formulas contains the same number of.

c2h5oh 염기 시보드
Lewis Structure of C2H5OH Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. This is a 2-D representation and it helps us to understand more about the properties of the compound. Let's move step-by-step and see how the Lewis Structure of C2H5OH can be made.
C2h5oh Lewis Dot Structure
C2H5OH (Ethanol) lewis structure has Carbon atoms (C) at the center which is surrounded by Hydrogen atoms (H) and one OH group. There are five C-H bonds, one O-H bond and one C-O bond. There are 2 lone pairs on the Oxygen atom (O).
C2h5oh Lewis Dot Structure
Use information from step 4 and 5 to draw the lewis structure. Lewis dot structure of C 2 H 5 OH. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C:4x2=8 H:1x6=6 O:6. Total=20 i.e . 10 pairs

C2h5oh Lewis Dot Structure
This structure will help us understand the properties of molecules along with their shape and molecular geometry. So first, we will determine the central atom for this molecule. Generally, the least electronegative atom takes the central position. Here Carbon is the least electronegative atom.

C2h5oh Lewis Dot Structure
Count the total number of electrons in the hypothetical structur e. If it is EQUAL to the number of available electrons from step 1, this is the correct Lewis Dot Structure. If it is GREATER than the number of available electrons from step 1, go to step 5. If it is LESS than the available number of electrons from step 1, go to step 7.

C2h5oh Lewis Dot Structure
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

C2H5OH Lewis Structure, Molecular Geometry, Hybridization, and Polarity
For the C2H5 + structure use the periodic table to find the total number of valence electrons for the C2H5 + molecule. Once we know how many valence electron.
Vieme chémiu
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".